Indications: Fenofibrate 200 mg tablets are indicated as an adjunct to diet for the following:
● Treatment of severe hypertriglyceridaemia with or without low HDL cholesterol.
● Mixed hyperlipidaemia when a statin is contraindicated or not tolerated.
● Mixed hyperlipidaemia in patients at high cardiovascular risk in addition to a statin when triglycerides and HDL cholesterol are not adequately controlled.
Pharmacological Properties:
Pharmacodynamics:
Fenofibrate is a pro-drug of the active chemical moiety fenofibric acid. Fenofibrate is converted by ester hydrolysis in the body to fenofibric acid which is the active constituent measurable in the circulation.
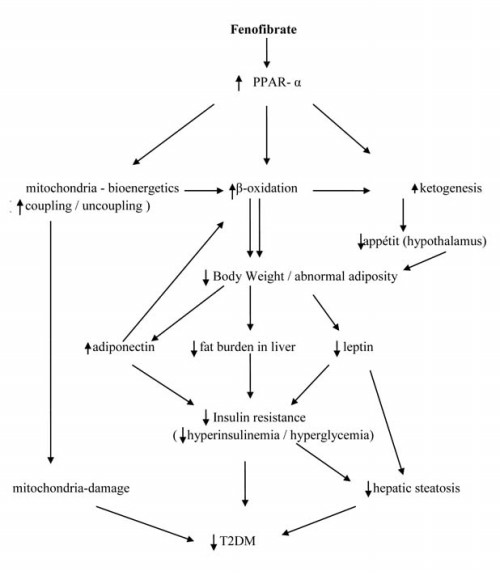
Fenofibric acid, the active metabolite of fenofibrate, produces reductions in total cholesterol, LDL cholesterol, apolipoprotein B, total triglycerides and triglyceride rich lipoprotein (VLDL) in treated patients. In addition, treatment with fenofibrate results in increases in high density lipoprotein (HDL) and apolipoproteins apoAI and apoAII.
It lowers lipid levels by activating Peroxisome proliferator-activated receptor alpha (PPARα). PPARα activates lipoprotein lipase and reduces apoprotein CIII, which increases lipolysis and elimination of triglyceride-rich particles from plasma.
Pharmacokinetics:
 Absorption: Maximum plasma concentrations (Cmax) occur within 4 to 5 hours after oral administration. Plasma concentrations are stable during continuous treatment in any given individual.
Absorption: Maximum plasma concentrations (Cmax) occur within 4 to 5 hours after oral administration. Plasma concentrations are stable during continuous treatment in any given individual.Distribution: Fenofibric acid is strongly bound to plasma albumin (more than 99%).
Plasma half-life: The plasma elimination half-life of fenofibric acid is approximately 20 hours.
Metabolism and Excretion: No unchanged fenofibrate can be detected in the plasma where the principal metabolite is fenofibric acid. The drug is excreted mainly in the urine. Practically all the drug is eliminated within 6 days. Fenofibrate is mainly excreted in the form of fenofibric acid and its glucuronide conjugate. In elderly patients, the fenofibric acid apparent total plasma clearance is not modified.
Interactions:
Oral anticoagulants: Fenofibrate enhances oral anticoagulant effect and may increase risk of bleeding.It is recommended that the dose of anticoagulants is reduced by about one third at the start of treatment and then gradually adjusted if necessary according to INR (International Normalised Ratio) monitoring. Therefore, this combination is not recommended
Ciclosporin: Some severe cases of reversible renal function impairment have been reported during concomitant administration of fenofibrate and ciclosporin. The renal function of these patients must therefore be closely monitored and the treatment with fenofibrate stopped in the case of severe alteration of laboratory parameters.
HMG-CoA reductase inhibitors and other fibrates: The risk of serious muscle toxicity I increased if fenofibrate is used concomitantly with HMG-CoA reductase inhibitors or other fibrates. Such combination therapy should be used with caution and patients monitored closely for signs of muscle toxicity.
Side Effects:
● Headache, Back pain
● Nasopharyngitis
● Nausea, Diarrhea
● Myalgia
● Joint pain or arthralgia
Warnings:
Renal function: Treatment should be interrupted in case of an increase in creatinine levels > 50% ULN (upper limit of normal). It is recommended that creatinine is measured during the first three months after initiation of treatment and thereafter periodically.
Liver function: As with other lipid lowering agents, increases have been reported in transaminase levels in some patients. In the majority of cases these elevations were transient, minor and asymptomatic. It is recommended that transaminase levels are monitored every 3 months during the first 12 months of treatment and thereafter periodically.
Muscle: Muscle toxicity, including rare cases of rhabdomyolysis, with or without renal failure has been reported with administration of fibrates and other lipid-lowering agents. The incidence of this disorder increases in cases of hypoalbuminaemia and previous renal insufficiency.
Storage:
Store in a cool, dry place, away from direct heat and light.